Osmosis
Created | Updated Nov 17, 2006
Most of us bump into this weird term when reading biology textbooks, particularly with reference to the parts concerning cell membranes. Osmosis1 is the flow of a solvent (usually water) through a semi-permeable membrane2 (like the cell membrane) in the direction of the concentrated solution. The osmotic flow is usually attributed to the natural tendency to balance both side's solute concentrations. The osmotic flow stops when the concentrations are balanced or when the hydrostatic pressure acting against this flow becomes as high. This process is illustrated in the figure below:
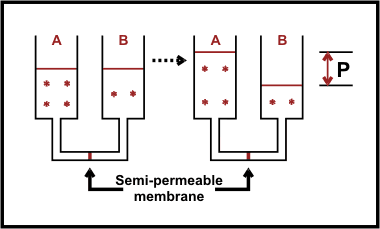
In the figure above the two compartments A and B are connected. In the connection pipe below the reservoirs the solutions are separated by a semi-permeable membrane. Compartment A has a higher concentration of solute than compartment B. To compensate against the osmotic pressure in A, the solvent from B will flow to A. This will cause the hydrostatic pressure in A to increase, and the osmotic pressure to decrease (the concentration also decreases). After a while balance is reached, namely when the hydrostatic pressure difference (P), which pushes solvent from A to B is equal to the osmotic pressure which pushes solvent from B to A.
As a result one can swell (or burst) cells by placing them in distilled water (the interior of the cell has a higher concentration of solutes) and shrink cells by placing them in concentrated salt solutions (the interior of the cells are less concentrated than the surrounding medium3).
In most cases this description is good enough to get the picture, but some notions in the description above are a bit over-simplified. A more precise description can be found in the following.
The Osmotic Pressure
The first description of an experiment involving osmosis was given in 1748 by a man named Jean-Antoine Nollet (1700-1770), a French priest and scientist. But it wasn't until 1885, when a more accurate description of this phenomenon was given by a Dutch fellow by the name of Jacobus Henricus van't Hoff4. He proposed that the dissolved particles in the solvent behave like ideal gas particles (see the Gas State Equations). According to this theory, the partial pressure (p) of these particles is given by the following equation. It was a modification of this simple equation that earned van't Hoff the chemistry Nobel prize in 1901:
p = (n/V)RT
Where the quotient (n/V) is the amount of particles per volume, or the molar concentration (c)5, R is the gas constant (8.314 J·K-1·mol-1) and T the temperature (which is constant in this process). So, simplifying, the partial pressure is directly proportional to the concentration:
p ~ c
Example: In a solution of 18 grammies of sucrose (n = 0.1 mol) in 1 litre of water, the partial pressure of the dissolved sucrose molecules will be 239 kPa (or roughly 2.3 atm).
This partial pressure is called osmotic pressure, when the compartment in which the solution is situated is separated by a semi-permeable membrane from another recipient containing the same solvent. So, if the concentration of dissolved particles rises then the osmotic pressure will be higher. Adding the osmotic pressure to the 'common' hydrostatic pressure of the solution will generate a higher overall pressure in the concentrated compartment. Why, then, does water flow in, apparently in the opposite direction, if the pressure is higher?
There are two ways to equilibrate the pressures on both sides, either let the solute diffuse to the less concentrated compartment (which would happen if there was no membrane) or dilute the compartment with the higher concentration at the expense of rising the hydrostatic pressure (which is the visible effect of the osmotic flow). In the end the pressure caused by the dissolved particles gets reduced, but the hydrostatic pressure increases. The point is, that the partial pressure decreases faster than the hydrostatic pressure increases, leading to a dynamic equilibrium after a while.
The osmotic pressure formula is not ultimately accurate, because it bases on the assumption of ideal behaviour. As we know from nature, things do not behave the way we want them to. So, the 'real measured' osmotic pressure is always a little higher than the calculated value. Generally, though, for solutions with a concentration below 1 mol/l the approximation is quite good.
Example: Sucrose in water at 293 K.
- Concentration 0.1 mol/l; osmotic pressure observed: 262 kPa; calculated: 239 kPa.
- Concentration 0.75 mol/l; osmotic pressure observed: 2.40 MPa; calculated: 1.87 MPa.
The Osmotic Potential
Many people are not used to the concept of osmotic pressure, since it is not a pressure one can feel with the finger or measure with a simple barometer. Again, osmosis needs a semi-permeable membrane. For that reason the term osmotic potential is widely used. The osmotic potential is defined as the capability of a solution to suck water in if it was separated from another solution by a semi-permeable membrane. For some weird reason it is always a negative number. So, the higher the negative number (the smaller the number, or the more negative) of the osmotic potential of a solution, the more it will suck water in, or the more concentrated it will be.
The terms isotonic, hypotonic and hypertonic describe the difference in osmotic pressure between two solutions with a certain osmotic potential. Two solutions are isotonic when the osmotic potentials are equal. When they are different, the one with the higher (read: less negative) potential will be hypotonic (less pressure) and the one with the lower potential (higher number or more negative) will be hypertonic (more pressure).
Applications of Osmosis
Osmotic pumps are widely used to generate vacuum. Semi-permeable membranes are used to obtain potable water from sea water, a case of inverse-osmosis. Some cells for instance intentionally don't compensate osmosis to the full extent therefore building up a electrochemical potential (in measured in Volts) which can be used to transmit electric signals (nerve-cells), or light lamps (electric eel).
Osmosis is not restricted to liquid solutions, it is a general phenomenon observable whenever there are semi-permeable membranes. The entrance of a bar, for instance, where only women are allowed to pass freely, will increase the pressure of the men on the outside. This is also a form of osmosis.
The Biological Importance of Osmosis
From the chapters before it becomes clear that osmosis is a phenomenon that takes place whenever there's a semi-permeable membrane. The cell membrane being one of such, makes osmosis one important phenomenon that must be taken into account by all living organisms. Plants use osmosis to increase turgor, and animal organisms need to control the osmotic pressure to avoid an over inflation and over shrinking of cells.
Plant cells have a cell wall surrounding them. It is made of cellulose, a sugar polymer (the stuff paper is made of). This wall is quite rigid and it prevents the cell from bursting due to osmosis. The solution in the inner part of a plant cell is normally more concentrated than the outside. For that reason, if a plant cell has good access to water the cells will be stiffened and filled with water (due to osmosis), making the whole plant rigid. If the water is more concentrated (eg salty water) then the plant will lose rigidity and wilt6.
Animal cells do not have the above mentioned cell wall. Many cells do have a so-called cytoskeleton, made out of proteins and small sugar chains in the inner part of the cell that are anchored to the cell membrane. In most cases this measure is enough to protect a cell from shrinking and bursting, but in a more narrow pressure range. For that reason many organisms are specialised to a certain environment, like fresh water or sweet water. More complex organisms have developed a skin, which roughly blocks the penetration of liquids from the outside to the inner part of the body. The fluids flowing in the inner part of an organism like the blood plasma must have a concentration of solutes that are within a very narrow range. This concentration is steered by specialised organs.

